Background: Myelofibrosis (MF) is a disorder characterized by unrestrained proliferation of myeloid precursors and dysfunctional Janus kinase (JAK) signaling. Fibroblast proliferation, bone remodeling, and marrow fibrosis within the osteohematopoietic niche (OHN) result in ineffective and extramedullary hematopoiesis. Clinical manifestations include anemia, splenomegaly, and often disabling symptoms. JAK inhibitors, such as ruxolitinib, have been shown to improve spleen size and symptoms but are associated with dose-limiting cytopenia, reducing their utility for many patients. KER-050 is an investigational, modified activin receptor type IIA ligand trap designed to inhibit select TGF-β superfamily ligands (activins A, B, GDFs 8, 11) to address ineffective hematopoiesis by promoting differentiation of erythroid and megakaryocytic precursors. Here, we present new data from an ongoing Phase 2 study (NCT05037760) evaluating KER-050 as a monotherapy and in combination with ruxolitinib in participants with MF.
Methods: This ongoing Phase 2 study is evaluating the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and efficacy of KER-050 administered with or without ruxolitinib in participants with anemia and primary MF, post-essential thrombocythemia MF, or post-polycythemia vera MF. Part 1 involves parallel dose escalation arms (1A: monotherapy, 1B: combination with ruxolitinib) to identify the dose(s) of KER-050 to be evaluated in Part 2. Data are presented as of the data cutoff date of April 3, 2023.
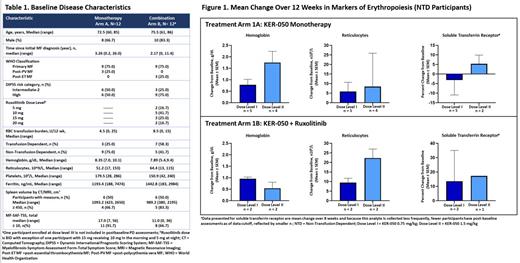
Results: A total of 24 participants were enrolled (n=12 in Arm 1A and n=12 in 1B). One participant recently enrolled in Arm 1B at dose level III (3.0 mg/kg) had limited exposure at the time of data cutoff and is not included in the PD findings reported below. All participants met the criteria for higher-risk disease by DIPSS and 41.7% met IWG 2013 transfusion-dependent (TD) criteria (Table 1). The median duration of KER-050 treatment was 116 days (range 26 to 474), with 62.5% of participants ongoing as of the cutoff date. Most participants (91.7%) had at least 1 treatment-emergent adverse event (TEAE). No dose-limiting toxicities and no treatment-related severe or serious TEAEs have been observed. Most frequently observed TEAEs in ≥15% of participants were fatigue (including asthenia) (37.5%), and diarrhea (20.8%). Mean increases in hemoglobin (Hgb), reticulocytes, and soluble transferrin receptor (sTfR) were observed over the first 12 weeks of treatment in non-TD (NTD) participants for whom changes in markers of erythropoiesis were less confounded by ongoing transfusions (Figure 1). Overall, 7 of 13 (53.8%) NTD participants achieved a mean Hgb increase of ≥1.0 g/dL over the first 12 weeks and 2 of 13 (15.4%) achieved an increase of ≥1.5 g/dL. Increases in Hgb were also observed among TD participants, with 3/10 (30%) having a Hgb increase ≥1 g/dL over the first 12 weeks and one participant achieving a transfusion reduction ≥50% over at least 12 weeks (data not shown). For this participant (dose level I, Arm 1B), transfusion burden decreased from 9 red blood cell (RBC) units/12 weeks at baseline to 2 RBC units/12 weeks. Another participant (dose level I, Arm 1B), who received 4 RBC units/12 weeks at baseline, achieved a 20-week transfusion-free period with concomitant increases in Hgb, sTfR and platelets. Across treatment arms, platelet values were generally maintained if not increased. Additionally, for one participant receiving KER-050 monotherapy (dose level I), spleen size decreased by 38% from 2569 cm 3 at baseline to 1592 cm 3 at 24 weeks, coinciding with a 55% reduction in MF-SAF-TSS score from 56 to 25.
Summary: Preliminary data from this ongoing Phase 2 study suggest that KER-050 is generally well tolerated and support the potential of KER-050 to improve key aspects of MF, including cytopenia, spleen size, and symptoms. Despite data being limited thus far to the 2 lowest dose levels, encouraging increases in markers of hematopoiesis have been observed as well as an initial case of reduced spleen size in a participant receiving KER-050 monotherapy. Updated analyses available at the time of presentation will provide further insight into the potential of KER-050 to treat MF and mitigate ruxolitinib-associated cytopenia.
Disclosures
Harrison:GSK: Honoraria, Speakers Bureau; CTI: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Speakers Bureau; AOP: Honoraria, Speakers Bureau; Galecto: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Morphosys: Honoraria, Speakers Bureau. Ross:Menarini: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Research Funding. Chee:Keros Therapeutics: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees. Tan:Keros Therapeutics: Research Funding. Devos:Incyte: Consultancy, Honoraria; AOP Pharma: Consultancy, Honoraria; BMS-Celgene: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria. Graham:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. McGinty:Keros Therapeutics: Current Employment. Pace:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Wang:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Jiang:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Bobba:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Dawson:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Rovaldi:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Hankin:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Grayson:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company; BioCryst Pharmaceuticals: Ended employment in the past 24 months. Cooper:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Salstrom:Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal